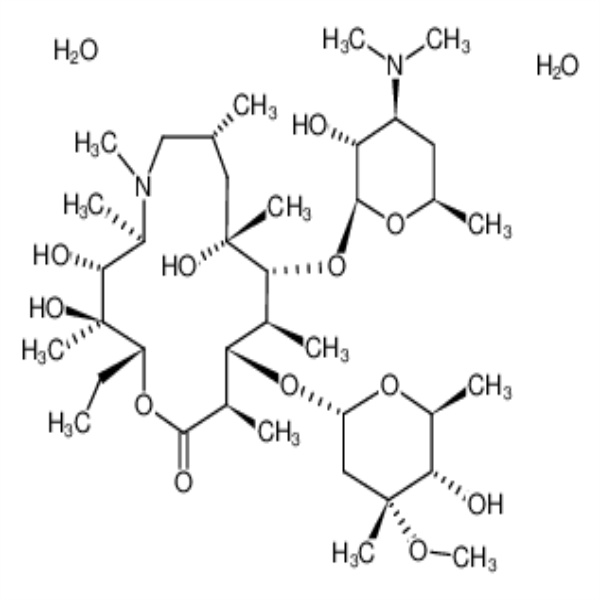
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Supply with High Purity and Stable Quality Chemical Name: Azithromycin Dihydrate CAS: 117772-70-0 API High Quality, Commercial Production| Item | Specifications |
| Appearance | White to Almost White Powder |
| Solubility | Freely soluble in Anhydrous Ethanol and in Methylene Chloride, Practically Insoluble in Water |
| Identification IR/HPLC | Conforms to Azithromycin reference standard |
| Crystallinity | Meets the Requirements |
| pH | 9.0~11.0 |
| Specific Optical Rotation | -45.0°~-49.0° |
| Heavy Metals | ≤20ppm |
| Water | 4.0~5.0% |
| Residue on Ignition | ≤0.20% |
| Related Substances | |
| Azithromycin-N-oxide | ≤0.50% |
| 3’-(N-N-Didemethyl)Azithromycin(aminozaithromycin) | ≤0.50% |
| Azithromycin Related Compound F | ≤0.50% |
| Desosaminylazithromycin | ≤0.30% |
| N-Demethylazithromycin | ≤0.70% |
| Azithromycin-C | ≤0.50% |
| 3’-De(dimethylamino)-3’-oxoazithromycin | ≤0.50% |
| Azaerythromycin A | ≤0.50% |
| Azithromycin Impurity P | ≤0.20% |
| 2-Desethyl-2-Propylazithromycin | ≤0.50% |
| 3’-N-DemETHYL-3’n-[(4-methylphenyl)sulfonyl]Azithromycin | ≤0.50% |
| Azithromycin-B | ≤1.0% |
| Any Unspecified Impurity | ≤0.20% |
| Total Impurities | ≤3.0% |
| Residual Solvents | |
| Methanol | ≤0.30% |
| Ethanol | ≤0.05% |
| Acetone | ≤0.50% |
| Chloroform | ≤0.006% |
| Assay | 945~1030μg/mg (C38H72N2O12 on anhydrous basis) |
| Shelf Life | 24 Months |
| Test Standard | USP Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | Azithromycin Dihydrate |
| CAS Number | 117772-70-0 |
| CAT Number | RF-API95 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C38H72N2O12 |
| Molecular Weight | 748.99 |
| Melting Point | 126℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Azithromycin is a broad-spectrum macrolide antibiotic with a long half-life and a high degree of tissue penetration 3. It was initially approved by the FDA in 1991. It is primarily used for the treatment of respiratory, enteric and genitourinary infections and may be used instead of other macrolides for some sexually transmitted and enteric infections. It is structurally related to erythromycin. Azithromycin prevents bacteria from growing by interfering with their protein synthesis. It binds to the 50S subunit of the bacterial ribosome, thus inhibiting translation of mRNA. Nucleic acid synthesis is not affected.