Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureManufacturer Supply, High Purity, Commercial Production| Item | Specifications |
| Appearance | White or Off-White Crystalline Powder |
| Identification | Correspond to Reference Standard |
| Solubility | Practically Insoluble in Water, Slightly Soluble in Ethanol |
| Purity / Analysis Method | >99.5% (HPLC) |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Heavy Metals | <10ppm |
| Related Substances | |
| Impurity A | <0.15% |
| Impurity B | <0.10% |
| Impurity C | <0.10% |
| Other Unknown Largest Single Impurity | <0.10% |
| Total Impurities | <0.50% |
| Residual Solvents | |
| Dichloromethane | <0.06% |
| Acetone | <0.50% |
| Ethyl Acetate | <0.50% |
| Methanol | <0.30% |
| Test Standard | Enterprise Standard |
| Usage | API |
Description:
Specifications:
Package & Storage:
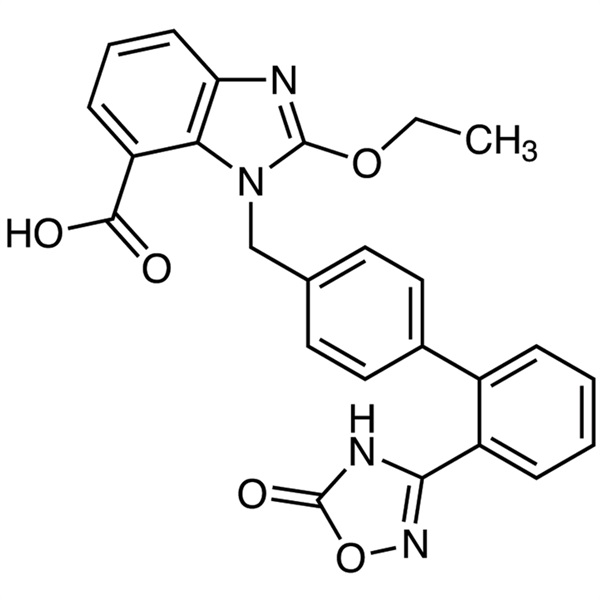
| Chemical Name | Azilsartan |
| Synonyms | 2-Ethoxy-1-[[2'-(4,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl]benzimidazole-7-Carboxylic Acid |
| CAS Number | 147403-03-0 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C25H20N4O5 |
| Molecular Weight | 456.46 |
| Melting Point | 188℃ (dec.) |
| Density | 1.42 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Azilsartan (CAS: 147403-03-0) is an angiotensin II receptor antagonist drug under development for the treatment of hypertension, which is mostly used for the treatment of hypertension. It is also the only angiotensin II receptor antagonist (sartan class) drug in the advanced clinical stage at present. Azilsartan and Chlorthalidone combination is used to treat high blood pressure (hypertension). Azilsartan is an angiotensin II receptor blocker (ARB). It works by blocking a substance in the body that causes blood vessels to tighten. As a result, azilsartan relaxes the blood vessels. This lowers blood pressure and increases the supply of blood and oxygen to the heart. Azilsartan was approved and launched in Japan for the treatment of arterial hypertension in May 2012. Azilsartan, which is marketed under the trade name Azilva.