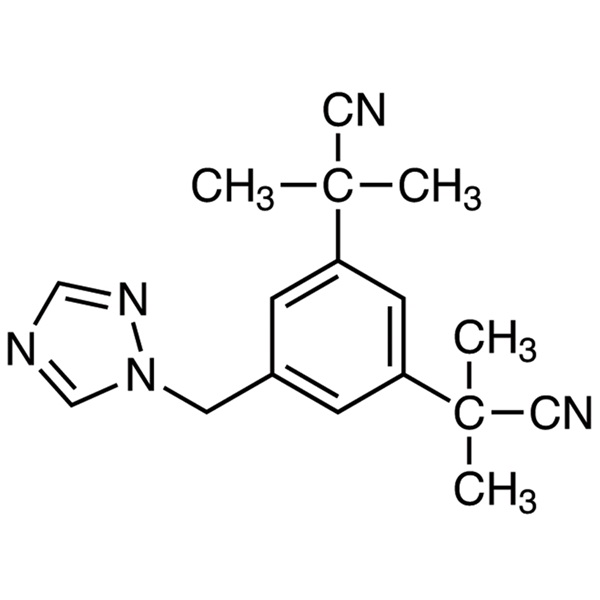
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer Supply with High Purity and Stable Quality Chemical Name: Anastrozole CAS: 120511-73-1| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification A | IR/MS Conform with reference standard |
| Identification B | HPLC and peak shape: Correspond to reference |
| Melting Point | 81.0~84.0℃ |
| Water Content (by K.F) | ≤0.30% |
| Residue on Ignition | ≤0.10% |
| Loss on Drying | ≤0.50% |
| Heavy Metals | ≤10ppm |
| Related Substances | |
| Impurity A | ≤0.10% |
| Impurity B | ≤0.20% |
| Impurity C | ≤0.20% |
| Impurity D | ≤0.10% |
| Impurity E | ≤0.10% |
| Single Other Impurity | ≤0.10% |
| Total Impurities | ≤0.50% |
| Residual Solvents | |
| Cyclohexane | ≤0.30% |
| Ethyl Acetate | ≤0.50% |
| Assay | 98.0%~102.0% (on the dried basis ) |
| Shelf Life | 24 Months |
| Test Standard | Enterprise Standard; United States Pharmacopoeia (USP) Standard |
| Usage | Treatment for Breast Cancer |
Description:
Specifications:
Package & Storage:
| Name | Anastrozole |
| Synonyms | Arimidex; α,α,α',α'-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile |
| CAS Number | 120511-73-1 |
| CAT Number | RF-API74 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C17H19N5 |
| Molecular Weight | 293.37 |
| Solubility | Soluble in DMSO and Ethanol |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Anastrozole (CAS 120511-73-1), is a non-steroidal, third-generation achiral triazole derivative marketed as ARIMIDEX® by AstraZeneca Pharmaceuticals LP1. It is one of the third-generation aromatase inhibitor which is a highly competitive and selective inhibitor of aromatase, thus blocking the conversion of testosterone into estradiol and androstenedione into estrone. Inhibition of the aromatase enzyme occurs particularly through competitive binding of aromatase to the hemegroup of cytochrome P450, decreasing estrogen biosynthesis in the peripheral tissues of the body and in the breast. Anastrozole is a generally well tolerated treatment for early-stage breast cancer. Like other aromatase inhibitors, its most important adverse effect was an increased risk of bone fractures, which for anastrozole was restricted to the treatment period. It characteristically has mild toxicity when compared with chemotherapy; however, it have been noticed that more patients treated with anastrozole have complained of joint symptoms than expected, particularly digital stiffness similar to that of rheumatoid arthritis. Some clinical trials of anastrozole for postmenopausal women with breast cancer in Europe and the United states reported musculoskeletal disorders as adverse events. Anastrozole (Arimidex®) is an aromatase inhibitor approved in the EU, the US and in other countries worldwide for use as an adjuvant treatment in postmenopausal women with early-stage, hormone receptor-positive breast cancer. It is also approved in the EU and other countries worldwide for continuing adjuvant treatment in women who have already had 2–3 years of adjuvant tamoxifen treatment for breast cancer.