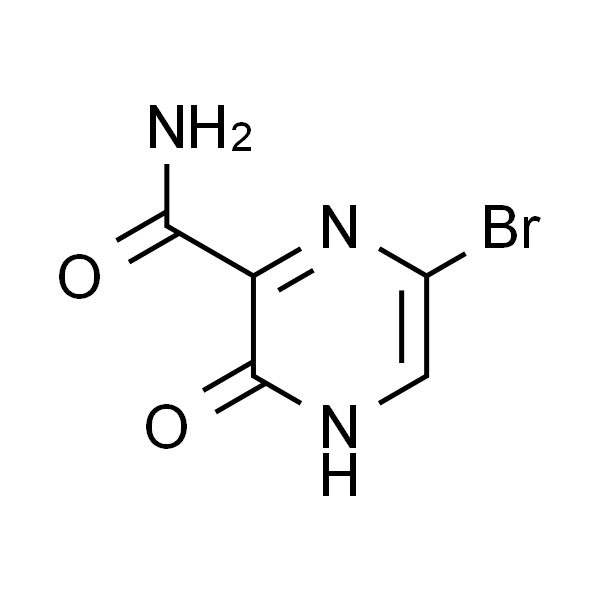
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer with High Purity and Stable Quality Commercial Supply Favipiravir and Related Intermediates: Favipiravir CAS 259793-96-9 2-Aminopropanediamide CAS 62009-47-6 Diethyl Aminomalonate Hydrochloride CAS 13433-00-6 3,6-Dichloropyrazine-2-Carbonitrile CAS 356783-16-9 3,6-Difluoropyrazine-2-Carbonitrile CAS 356783-28-3 6-Fluoro-3-Hydroxypyrazine-2-Carbonitrile CAS 356783-31-8 6-Bromo-3-Hydroxypyrazine-2-Carboxamide CAS 259793-88-9 3-Hydroxypyrazine-2-Carboxamide CAS 55321-99-8| Item | Specifications |
| Appearance | Light Yellow to Brown Powder |
| Identification | IR, HPLC |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.20% |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Heavy Metals | ≤20ppm |
| Residual Solvents | Meet the specification |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Favipiravir (CAS 259793-96-9) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 6-Bromo-3-Hydroxypyrazine-2-Carboxamide |
| Synonyms | Favipiravir Intermediate; Favipiravir Impurity 4 |
| CAS Number | 259793-88-9 |
| CAT Number | RF-PI296 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C5H4BrN3O2 |
| Molecular Weight | 218.01 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
6-Bromo-3-Hydroxypyrazine-2-Carboxamide (CAS 259793-88-9) is an intermediate typically in the synthesis of 6-Bromo-3-Hydroxypyrazine-2-Carboxamide commercially named Favipiravir (CAS 259793-96-9) in the treatment of Influenza virus infections. A practical and step-economic route to Favipiravir. Favipiravir is a COVID19-related research product, in the treatment of patients with COVID-19 recommended by WHO. Favipiravir, also known as T-705 or Avigan, is an antiviral drug being developed by Toyama Chemical (Fujifilm Group) of Japan with activity against many RNA viruses. Favipiravir was approved for marketing in Japan in March 2014 and used for antiviral treatment of influenza A and B. In addition to the influenza virus, the drug also showed good antiviral activity to a variety of RNA viruses, such as Ebola virus, sand virus, Bunia virus, rabies virus, etc. In February 2020 Favipiravir was being studied in China for experimental treatment of the emergent COVID-19 (Novel Coronavirus) disease and it has show positive results.