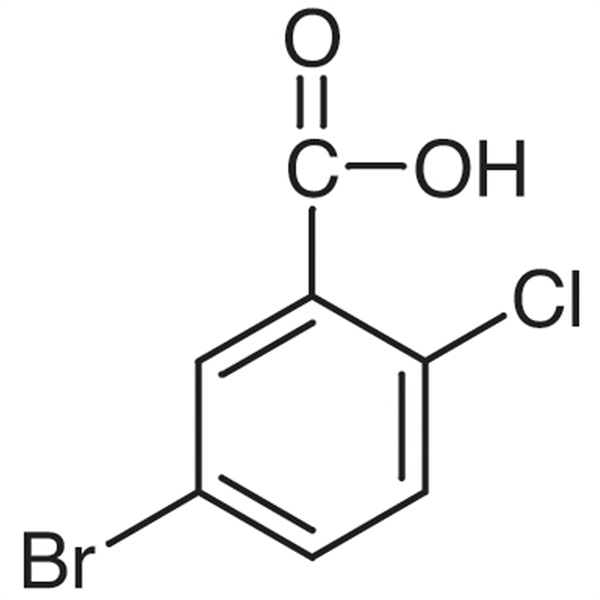
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply, High Purity, Commercial Production Dapagliflozin (CAS: 461432-26-8) Related Intermediates: 5-Bromo-2-Chlorobenzoic Acid CAS 21739-92-4 5-Bromo-2-Chloro-4'-Ethoxydiphenylmethane CAS 461432-23-5 2,3,4,6-Tetrakis-O-Trimethylsilyl-D-Gluconolactone CAS 32384-65-9| Item | Specifications |
| Appearance | Off-White to Light Yellow Powder |
| Assay | ≥99.0% |
| Water (K.F) | ≤0.50% |
| Residue on Ignition | ≤0.30% |
| 2-Chlorobenzoic Acid | ≤0.30% |
| 2-Chloro-3-Bromobenzoic Acid | ≤0.30% |
| Total Impurities | ≤1.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Dapagliflozin (CAS: 461432-26-8), type II diabetes |
Description:
Specifications:
Package & Storage:
| Chemical Name | 5-Bromo-2-Chlorobenzoic Acid |
| Synonyms | 2-Chloro-5-Bromobenzoic Acid |
| CAS Number | 21739-92-4 |
| CAT Number | RF-PI439 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C7H4BrClO2 |
| Molecular Weight | 235.46 |
| Melting Point | 154.0 to 156.0℃ (lit.) |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
5-Bromo-2-Chlorobenzoic Acid (CAS: 21739-92-4) is used as a starting material in the synthesis of Dapagliflozin (CAS: 461432-26-8), a selective sodium-glucose cotransporter-2 inhibitor that reduces renal glucose reabsorption and is used to treat patients with type II diabetes. Dapagliflozin is a potent and selective SGLT-2(SLC5A2 )inhibitor with EC50 of 1.1 nM. It has good permeability across Caco-2 cell membranes and is a substrate for P-glycoprotein (P-gp). Dapagliflozin was approved in 2012 by FDA.