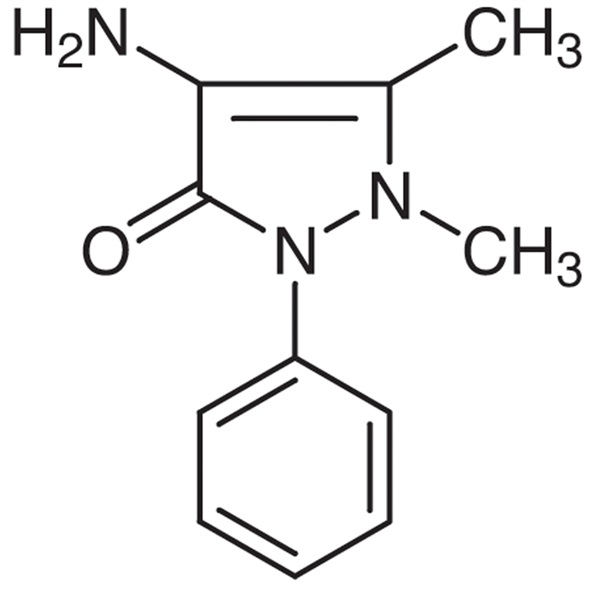
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 4-Aminoantipyrine (CAS: 83-07-8) with high quality, commercial production. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | Pale Yellow to Yellow-Brown Crystalline Powder |
| Purity | >99.0% |
| Melting Point | 106.0~110.0℃ |
| Loss on Drying | <1.50% |
| Sulfated Ash | <0.10% |
| Total Impurities | <1.00% |
| Solubility | Clear Yellow Liquid, 50mg/ml in Water |
| Infrared Spectrum | Conforms to Structure |
| NMR | Consistent With Structure |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | 4-Aminoantipyrine |
| Synonyms | 4-Aminophenazone; 4-AA; Ampyrone; NSC-6242; Metapirazone; Solnapyrin-A; Metamizole EP Impurity B |
| CAS Number | 83-07-8 |
| CAT Number | RF-PI1960 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C11H13N3O |
| Molecular Weight | 203.24 |
| Solubility | Soluble in Water, 500 g/L (20℃) |
| Hydrogendioxide Detection Test | To Pass Test (Peroxidase Method) |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
4-Aminoantipyrine (CAS: 83-07-8) shows both analgesic and anti-inflammatory properties. 4-Aminoantipyrine is used as a reagent for glucose determination in the presence of peroxidase and phenol. It is used as a drug dipyrone. 4-Aminoantipyrine is a metabolite of aminopyrine, having both analgesic and anti-inflammatory properties. Coupling Reagent for Trinder’s reagents in colorimetric hydrogen peroxide detectionassays Forms highly stable dyes by coupling with Trinder’s reagents in presence of Peroxidase and H2O2. Therefore suitable for use in test strip and solution diagnostics. 4-Aminoantipyrine is the most widely used analytical reagent for the estimation of phenol. 4-Aminoantipyrine forms heterocyclic Schiff bases, by reaction with various aldehydes and oximes. These Schiff bases form stable complexes with transition metals. 4-Aminoantipyrine is an intermediate of the Metamizole Sodium.