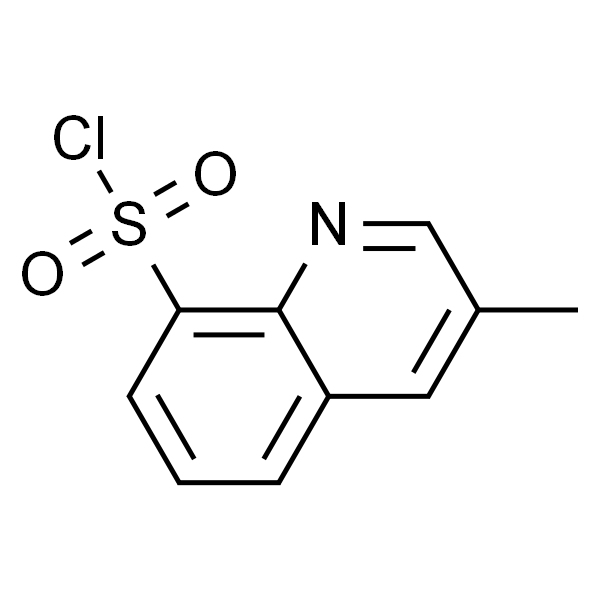
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light & moistureManufacturer Supply Argatroban Related Intermediates: Ethyl (2R,4R)-4-Methyl-2-Piperidinecarboxylate CAS 74892-82-3 N-Nitro-1,2,3,4-tetradehydro Argatroban Ethyl Ester CAS 74874-09-2 3-Methyl-8-Quinolinesulphonyl Chloride CAS 74863-82-4 Argatroban Monohydrate CAS 141396-28-3 Argatroban Anhydrous CAS 74863-84-6| Item | Specifications |
| Appearance | White Crystalline Powder |
| Purity / Analysis Method | >98.0% (HPLC) |
| Melting Point | 162.0~163.0℃ |
| Loss on Drying | <0.50% |
| 3-Methylquinoline-8-Sulfonic Acid | <1.00% |
| Total Impurities | <2.00% |
| Infrared Spectrum | Conforms to Structure |
| NMR | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Argatroban (CAS 74863-84-6) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 3-Methyl-8-Quinolinesulphonyl Chloride |
| Synonyms | 3-Methylquinoline-8-Sulfonyl Chloride |
| CAS Number | 74863-82-4 |
| CAT Number | RF-PI269 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H8ClNO2S |
| Molecular Weight | 241.69 |
| Density | 1.4±0.1 g/cm3 |
| Refractive Index | 1.63 |
| Solubility | Soluble in Chloroform |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 3-Methyl-8-Quinolinesulphonyl Chloride (CAS: 74863-82-4) with high quality, widely used in organic synthesis, synthesis of pharmaceutical intermediates and Active Pharmaceutical Ingredient (API) synthesis. It is an intermediate typically in the synthesis of Argatroban (CAS: 74863-84-6) or Argatroban Monohydrate (CAS 141396-28-3). Argatroban (CAS: 74863-84-6) is an anticoagulant that is a small molecule direct thrombin inhibitor. In 2000, argatroban was licensed by the Food and Drug Administration (FDA) for prophylaxis or treatment of thrombosis in patients with heparin-induced thrombocytopenia (HIT). In 2002, it was approved for use during percutaneous coronary interventions in patients who have HIT or are at risk for developing it. In 2012, it was approved by the MHRA in the UK for anticoagulation in patients with heparin-induced thrombocytopenia Type II (HIT) who require parenteral antithrombotic therapy.