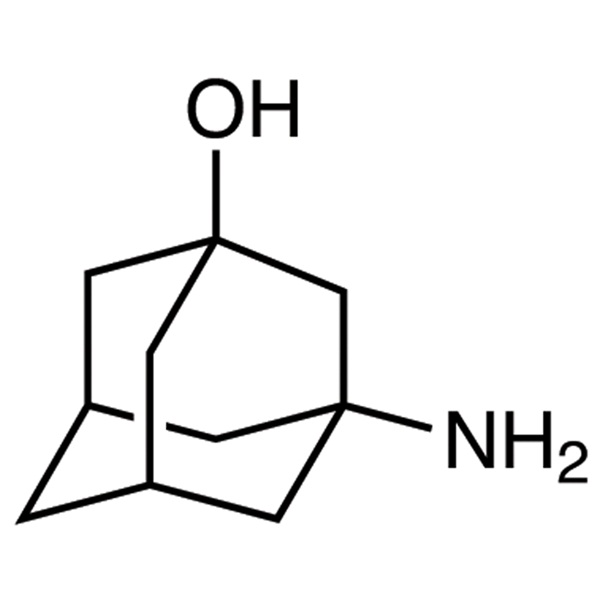
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light & moistureManufacturer Supply Related Intermediates: CAS: 274901-16-5 3-Amino-1-Adamantanol CAS: 702-82-9 L-Prolinamide CAS: 7531-52-4 Boc-L-Proline CAS: 15761-39-4 (2S)-1-(Chloroacetyl)-2-Pyrrolidinecarbonitrile CAS: 207557-35-5| Item | Specifications |
| Appearance | White Crystalline Powder |
| Purity / Analysis Method | >99.0% (HPLC) (dry basis) |
| Water (by Karl Fischer) | <0.50% |
| Residue on Ignition | <0.50% |
| Melting Point | 266.5℃~272.5℃ |
| Single Impurity | <0.50% |
| Total Impurities | <1.00% |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of API (CAS: 274901-16-5) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 3-Amino-1-Adamantanol |
| Synonyms | 1-Amino-3-Hydroxyadamantane; 3-Amino-1-Hydroxyadamantane |
| CAS Number | 702-82-9 |
| CAT Number | RF-PI102 |
| Stock Status | In Stock, Production Capacity 800MT/Year |
| Molecular Formula | C10H17NO |
| Molecular Weight | 167.25 |
| Solubility | Soluble in Organic Solvents and Insoluble in Water |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 3-Amino-1-Adamantanol (CAS: 702-82-9) with high quality. API (CAS: 274901-16-5) is synthesised from 3-Amino-1-Adamantanol. API (CAS: 274901-16-5) is an oral anti-hyperglycemic agent (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. API (CAS: 274901-16-5) inhibits the inactivation of GLP-1 and GIP by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucagon release by the alpha cells of the islets of Langerhans in the pancreas. It has been shown to reduce hyperglycemia in type 2 diabetes mellitus. It was approved in Feb 2008 by European Medicines Agency for use within the EU and is listed on the Australian PBS with certain restrictions.