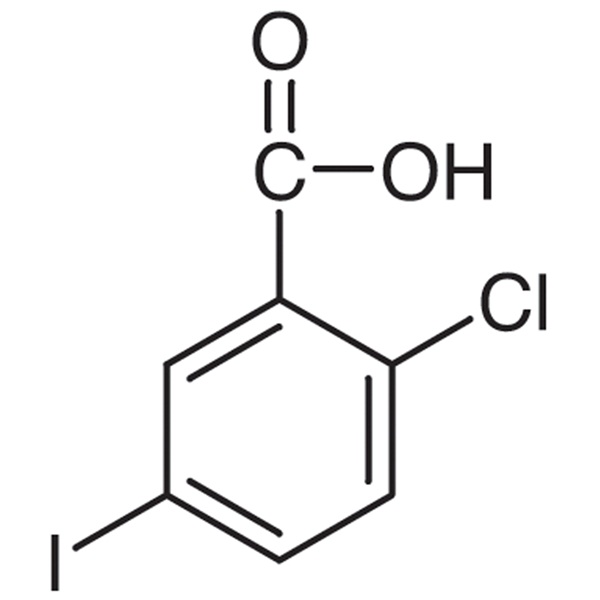
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply, High Purity, Commercial Production Chemical Name: 2-Chloro-5-Iodobenzoic Acid CAS: 19094-56-5| Item | Specifications |
| Appearance | White or Pale Yellow Crystals |
| Assay / Analysis Method | ≥99.0% (HPLC) |
| Melting Point | 161.0~163.0℃ |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.20% |
| Total Impurities | ≤1.0% |
| Heavy Metals | ≤20ppm |
| Residual Solvent | Ethanol |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Empagliflozin (CAS: 864070-44-0) |
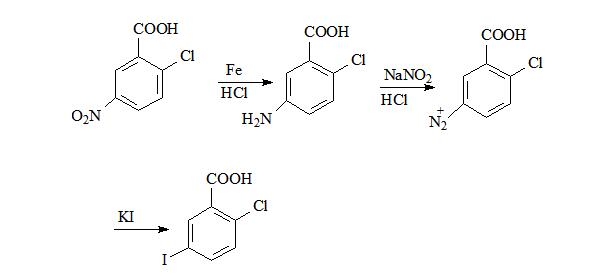
Description:
Specifications:
Package & Storage:
| Chemical Name | 2-Chloro-5-Iodobenzoic Acid |
| CAS Number | 19094-56-5 |
| CAT Number | RF-PI438 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C7H4ClIO2 |
| Molecular Weight | 282.46 |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
2-Chloro-5-Iodobenzoic Acid (CAS: 19094-56-5) can be used as a pharmaceutical intermediate in the synthesis of Empagliflozin (CAS: 864070-44-0). Empagliflozin (trade name Jardiance) is an inhibitor of the sodium glucose co-transporter-2 (SGLT-2), and causes sugar in the blood to be excreted by the kidneys and eliminated in urine. On August 1, 2014, the Food and Drug Administration (FDA) officially approved the drug for the treatment of type 2 diabetes, to improve and control blood glucose of adults. Empagliflozin is the third SGLT-2 inhibiting drugs approved by FDA. Another two SGLT-2 inhibitor drugs, Canagliflozin and Dapagliflozin, belonging to Johnson Pharmaceuticals, AstraZeneca and Bristol-Myers Squibb respectively, are approved by FDA in November 2013 and January 2014 respectively.