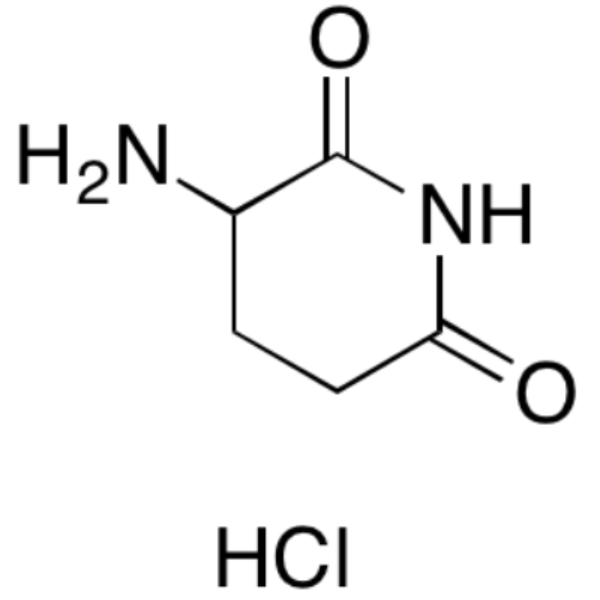
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 2,6-Dioxopiperidine-3-Ammonium Chloride (CAS: 24666-56-6) with high quality, commercial production. Welcomed to order.| Item | Specifications |
| Appearance | Off-White Crystalline Powder |
| Purity / Analysis Method | >99.0% (HPLC) |
| Moisture (K.F) | <0.50% |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Total Impurities | <1.00% |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Lenalidomide (CAS: 191732-72-6) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 2,6-Dioxopiperidine-3-Ammonium Chloride |
| Synonyms | 3-Aminopiperidine-2,6-Dione Hydrochloride;3-Amino-2,6-Piperidinedione Hydrochloride |
| CAS Number | 24666-56-6 |
| CAT Number | RF-PI1616 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C5H9ClN2O2 |
| Molecular Weight | 164.59 |
| Melting Point | 120℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
2,6-Dioxopiperidine-3-Ammonium Chloride (CAS: 24666-56-6) is an intermediate for preparing Lenalidomide (CAS: 191732-72-6). Lenalidomide is a kind of antitumor drugs that developed by American biological pharmaceutical companies. It has many functions such as anti-tumor, immune regulation and anti-angiogenesis. It can inhibit the secretion of inflammatory cytokines, and increase the secretion of peripheral blood mononuclear anti-inflammatory cytokines. It can inhibit the growth of patients’ multiple myeloma cells and MM1S cell. Two multicenter randomized double-blind placebo-controlled clinical studies evaluate the safety and curative effect of lenalidomide that is used for multiple myeloma. Recent clinical research results show that lenalidomide not only has curative effect on treating MDS and MM, but also on treating myeloma, leukemia, metastatic renal cell carcinoma, solid tumor, idiopathic generalized amyloidosis and systemic bone marrow fibrosis disease with marrow unripe. In December 2005, the US Food and Drug Administration (FDA) approved lenalidomide to be used in the treatment of myelodysplastic syndrome (MDS).